Neo-adjuvant ADT prior to RP: Are we there yet?
- Mar 5, 2024
- 2 min read
Dr Dharmender Aggarwal, Fortis Hospital, Mohali.
The rationale behind neo-adjuvant ADT in patients with high risk localized disease prior to definitive treatment is to downstage the local tumour as well as address the occult micro-metastatic disease. At the same time, it also allows to assess the in-vivo response of the disease to the systemic agents, similar to neo-adjuvant therapies used in other malignancies such as the urinary bladder.
Several systemic therapies, which are a standard of care for metastatic prostate cancer, are being tried in the neo-adjuvant settings also. Traditionally, conventional ADT in the form of LHRH analogues were tried in the neo-adjuvant settings and more recently the novel anti-hormonal agents such as enzalutamide, apalutamide or abiraterone are being tried in combination with LHRH analogues. Most of the older trials using conventional ADT showed a reduced positive margin rate and a higher likelihood of organ confined disease with neo-adjuvant ADT but did not find any difference in the rate of BCR or cancer-specific survival. However, these studies had a short follow up and included low/intermediate risk patients also and were not exclusive to high risk group. Also, it is believed, that the low lethal potential of conventional ADT contributed to the lack of survival benefit, as ypT0 was seldom achieved. With the advent of novel anti-hormonal agents, there is renewed interest in neo-adjuvant ADT prior to RP. Recent studies which combined abiraterone or enzalutamide with LHRH analogues in the neo-adjuvant settings showed a significant reduction in the intra-tumoral testosterone and tumour volume as compared to LHRH analogues alone and a significantly higher percentage of patients could achieve ypT0, thus establishing superiority and renewed interest.
Recent data suggests that neoadjuvant ADT improves pathological as well as oncological outcomes. Ravi P et al, in 2022, compared the outcomes of patients who received neo-adjuvant therapy with novel anti-hormonal agents prior to RP to those without neoadjuvant therapy.(1) After balancing for confounding factors, they found that the time to BCR and metastasis free survival was significantly longer in the neo-adjuvant group. Also, the rate of adjuvant treatment and salvage therapy were lower in neo-adjuvant group.
Despite the promising recent evidence, neo-adjuvant ADT prior to RP has not yet become the standard of care. Proteus trial, which is an undergoing double-blind randomized control trial, is going to assess the benefits and side effects of combination ADT prior to RP for locally advanced or high risk localised prostate cancer, and the results of this trial are eagerly awaited to settle the role of neo-adjvant ADT prior to RP.
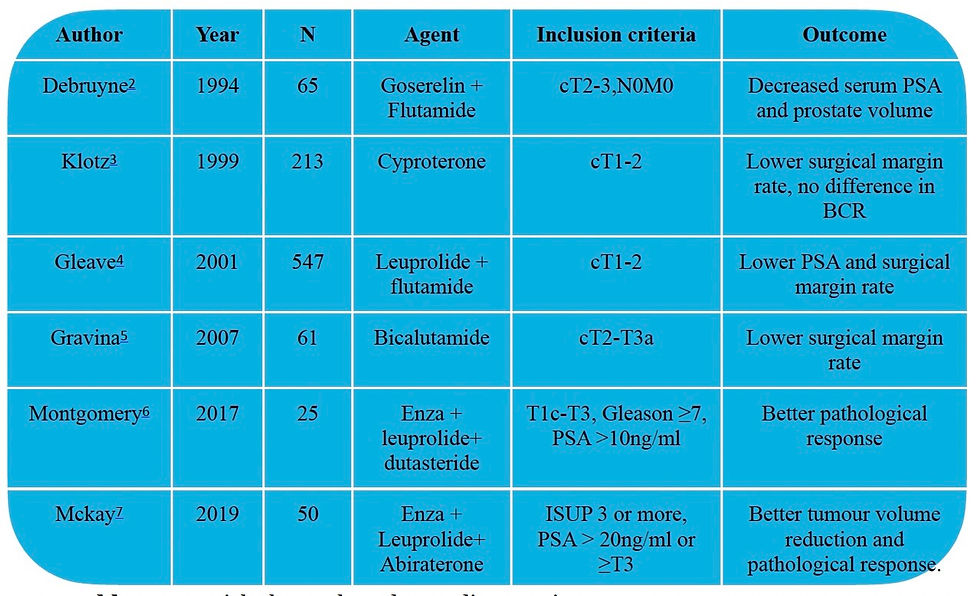
Author | Year | N | Agent | Inclusion criteria | Outcome |
Debruyne2 | 1994 | 65 | Goserelin + Flutamide | cT2-3,N0M0 | Decreased serum PSA and prostate volume |
Klotz3 | 1999 | 213 | Cyproterone | cT1-2 | Lower surgical margin rate, no difference in BCR |
Gleave4 | 2001 | 547 | Leuprolide + flutamide | cT1-2 | Lower PSA and surgical margin rate |
Gravina5 | 2007 | 61 | Bicalutamide | cT2-T3a | Lower surgical margin rate |
Montgomery6 | 2017 | 25 | Enza + leuprolide+ dutasteride | T1c-T3, Gleason ≥7, PSA >10ng/ml | Better pathological response |
Mckay7 | 2019 | 50 | Enza + Leuprolide+ Abiraterone | ISUP 3 or more, PSA > 20ng/ml or ≥T3 | Better tumour volume reduction and pathological response. |
Table 2: Few trials that evaluated neo-adj ADT prior to RP




Comments